These services include physiologic stress testing, non-invasive cardiac imaging, platelet aggregation, ambulatory blood pressure monitoring, and other services in addition to the QT tests. Due to the grouping of services with an emphasis on end-to-end development, integrated services that provide cardiac safety services are generally favored over standalone services because of the peculiarity of standalone services.
All elements of the cardiovascular system, including the heart, blood vessels, and blood constituents, are affected by cardiovascular safety liabilities, which include both cardiovascular and non-cardiovascular pharmaceuticals. Cardiovascular adverse effects can be functional or structural (such as histopathology) in nature and can happen after acute or chronic treatment.
The need for effective yet secure drug development is more critical than ever in a time of growing public scrutiny, rising business expenses, and limited resources at regulatory agencies. With 17.9 million deaths per year, cardiovascular diseases (CVDs) are the most common cause of death worldwide. Cerebrovascular disease, coronary heart disease, rheumatic heart disease, and other illnesses are among the category of heart and blood vessel disorders known as CVDs.
COVID-19 Impact Analysis
This prompted the pharmaceutical and health insurance industries to make the necessary changes to their offerings in order to include better provisions and facilities. In order to progress the evolution of medicine, this compelled the engagement of numerous research institutions and organizations dedicated to heart health. Coronavirus-related catastrophes caused a significant number of patients with pre-existing unfavorable disorders to succumb to them, which had an effect on cardiac safety services. As a result of COVID-19's vigorous promotion of its development, the market for cardiac safety services was positively affected overall.Market Growth Factors
Increase In Research For Biosimilars And Biologics
Numerous businesses are making significant investments in the creation of biologics and biosimilar compounds. In the discovery stage, biologics such peptides, proteins, and monoclonal antibodies make up more than half of the therapeutic candidates. Pharmaceutical and biopharmaceutical businesses are heavily putting money into their research and development as novel biologics are being developed or are in the pipeline. Biosimilars are also less expensive because, being generic versions of patented biologic medications, they are not subject to the same strict regulatory standards.High Prevalence Of Ech Holter Service Among The General Population
The rise in the number of elderly people and the increasing incidence of cardiovascular disorders are mostly to blame for the ECG Holter's expansion. According to data released by Health System Tracker, heart disease is the top cause of mortality in the United States. These reasons make immediate heartbeat detection and constant heart rate monitoring equipment necessary. In the past few years, the healthcare system has employed ECG monitoring devices to look for any abnormalities in cardiac activity.Market Restraining Factors
Lack Of Qualified Personnel For Clinical Trials
The R&D outsourcing industry for pharmaceuticals, biotechnology, and medical devices is always changing. To deliver high-quality services, adhere to acceptable laboratory procedures, and keep up with the ongoing developments in medical device and pharmaceutical R&D technologies and techniques, highly skilled experts are needed. As they compete for trained and experienced scientists with biotechnology, pharmaceutical, and medical device businesses as well as research and academic organizations, CROs confront difficulties in attracting and keeping highly skilled personnel.Type Outlook
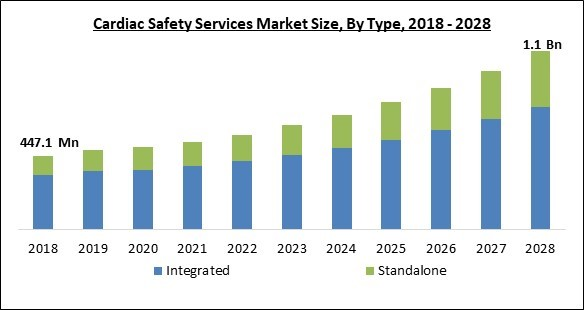
Based on type, the cardiac safety services market is bifurcated into integrated and standalone. The integrated segment dominated the cardiac safety services market with the highest revenue share in 2021. These cutting-edge core lab services provide a full range of cardiac safety services, including imaging, TQT, and profile QT tests. In order to help with the real-time evaluation of heart rate and rhythm, they also keep an eye on off-target cardiovascular liabilities and conduct onsite multichannel telemetry under the supervision of licensed nurses.Type of Service Outlook
On the basis of type of service, the cardiac safety services market is divided into ECG/Holter measurement, blood pressure measurement, cardiovascular imaging, thorough QT studies, and other services. The ECG/Holter measurement segment witnessed the largest revenue share in the cardiac safety services market in 2021. Smartwatches and other personal electronics provide electrocardiogram monitoring. A small, portable gadget called a Holter monitor is used to capture heartbeats. It is employed to identify and evaluate the likelihood of aberrant heartbeats (arrhythmias).End User Outlook
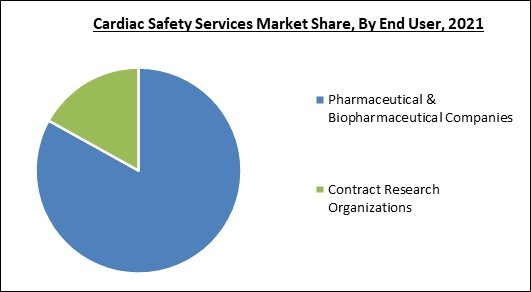
Based on end user, the cardiac safety services market is categorized into pharmaceutical & biopharmaceutical companies and contract research organizations. The contract research organizations segment procured a substantial revenue share in the cardiac safety services market in 2021. Clinical trials are carried out by Contract Research Organizations (CROs) for the pharmaceutical, medical device, and biotechnology businesses as well as for academic institutions, governmental agencies, and foundations.Regional Outlook
On the basis of region, the cardiac safety devices is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region acquired the highest revenue share in the cardiac safety services market in 2021. This is due to the region's growing number of clinical studies, which are fueling demands for cardiac safety services. The rising number of people suffering from cardiovascular diseases (CVD) and coronary heart disease (CHD) have accelerated the drug development and research studies in the region.Cardinal Matrix-Cardiac Safety Services Market Competition Analysis
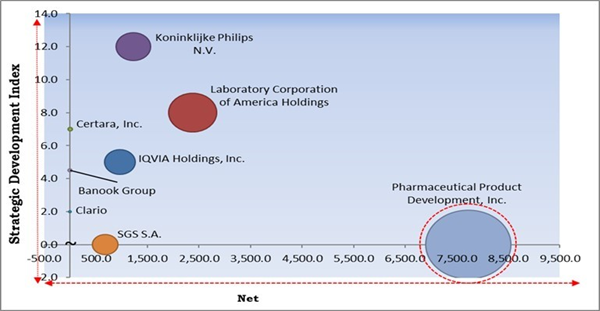
The major strategies followed by the market participants are Acquisitions. Based on the Analysis presented in the Cardinal matrix; Pharmaceutical Product Development, Inc. is the major forerunner in the Cardiac Safety Services Market. Companies such as Koninklijke Philips N.V., Laboratory Corporation of America Holdings, IQVIA Holdings, Inc. are some of the key innovators in Cardiac Safety Services Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Koninklijke Philips N.V., Clario, IQVIA Holdings, Inc., Laboratory Corporation of America Holdings, Pharmaceutical Product Development, Inc., SGS S.A., Banook Group, Biotrial Research SAS, Certara, Inc., and Celerion.
Strategies deployed in Cardiac Safety Services Market
- Aug-2022: Labcorp acquired RWJBarnabas Health, a network of independent healthcare providers in New Jersey. Through this acquisition, Labcorp planned to offer expanded health plan coverage, enhanced service to rural markets, and the potential for reduced out-of-pocket lab costs for patients.
- Jun-2022: The Banook Group expanded its geographical footprint by opening its first US office in Boston, Massachusetts. Through this expansion, Banook aimed to reduce the constraints of time zones and to provide enhanced technical and logistics support to its customers as about 40% of Banook’s customers are based in this region. The expansion also allowed the company to offer local support to its 70 current US customers and to grow in the North American market.
- Apr-2022: Royal Philips, partnered with Prisma Health, South Carolina's largest non-profit healthcare system. Through this partnership, the companies agreed to help the health system achieve enterprise interoperability, standardize patient monitoring, and drive innovation in enterprise imaging solutions to enhance patient care and improve clinical performance.
- Mar-2022: IQVIA unveiled OCE+, the first of several new advancements to its leading life science customer engagement platform. OCE+ adds IQVIA’s Next Best recommendation engine to its Orchestrated Customer Engagement (OCE) platform, providing enhanced HCP experiences, improved productivity, and increased ROI. The product uses IQVIA’s industry-leading data and advanced analytics to deliver AI-driven recommendations directly into the daily workflows of life sciences commercial teams.
- Jan-2022: Clario partnered with ActiGraph, a pioneer and leading provider of activity-sensing wearable technology. From this partnership, Clario expanded its evidence generation platform and portfolio of decentralization technologies and offered ActiGraph technologies to its customers in addition to its suite of eCOA, Precision Motion, Cardiac Safety, Respiratory, Medical Imaging, and Trial Enablement solutions. The partnership also helped clinical trial sites and sponsors to drive up efficiency and accuracy while increasing convenience for study participants.
- Dec-2021: Labcorp took over Personal Genome Diagnostics (PGDx), a provider of comprehensive liquid biopsy and tissue-based genomic products and services. From this acquisition, Labcorp aimed to strengthen its next-generation sequencing-based genomic profiling capabilities and accelerate the existing liquid biopsy capabilities.
- Nov-2021: Royal Philips acquired Cardiologs, a French medical technology firm. From this acquisition, Royal Philips aimed at expanding its cardiac monitoring and diagnostics portfolio with innovative software technology, electrocardiogram (ECG) analysis, and reporting services.
- Sep-2021: The Banook Group acquired Nabios, biotech with strong expertise in cardiac safety assessments in clinical trials of drugs intended for human health. Through this acquisition, the company advanced its steps towards becoming the leader in the cardiac safety market by offering a strong European alternative to those companies operating from North America or India. This acquisition also granted Banook's customers access to new and more competitive services.
- Sep-2021: IQVIA collaborated with HealthCore, a leading real-world research organization in the United States. Under the collaboration, the companies focused on advancing real-world evidence (RWE) studies with increased quality and efficiency. The innovative research collaboration focused specifically on real-world studies such as external comparators, pragmatic trials, and enriched studies.
- Mar-2021: Certara took over AUTHOR!, a provider of medical writing and statistical analysis of clinical trial data to global pharmaceutical and biotechnology companies. Through this acquisition, Certara enhanced the existing regulatory and biostatistical expertise and helped to fuel the company's strategic expansion in Europe. Additionally, from the acquisition, Certara leveraged AUTHOR!’s seasoned team to provide technology-enabled capabilities to its customers and thus help global clients expertly navigate and accelerate the drug development and regulatory approval process.
- Feb-2021: Royal Philips took over BioTelemetry, a leading U.S.-based provider of remote cardiac diagnostics and monitoring. Through this acquisition, Royal Philips expanded its cardiac care portfolio, and its strategy to transform the delivery of care along the health continuum with integrated solutions.
- Feb-2021: Philips formed a partnership with SAZ hospital network, a Dutch Hospital network comprising 28 hospitals. Under this partnership, Philips offered advanced patient monitoring and population health management solutions to the SAZ network hospitals. In addition, Philips solutions enabled SAZ to enhance monitoring, observation, and self-management of patients across the care journey, both inside and outside the hospitals.
- Mar-2018: Certara introduced Quantitative Systems Pharmacology (QSP) Immuno-oncology Simulator Consortium. The product combines computational modeling and experimental methods to examine the mechanistic relationships between a drug, the biological system, and the disease process. In addition, QSP integrates quantitative drug data with the knowledge of the drug’s mechanism of action.
Scope of the Study
Market Segments Covered in the Report:
By Type
- Integrated
- Standalone
By End User
- Pharmaceutical & Biopharmaceutical Companies
- Contract Research Organizations
By Type of Service
- ECG/Holter Measurement
- Blood Pressure Measurement
- Cardiovascular Imaging
- Thorough QT Studies
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Koninklijke Philips N.V.
- Clario
- IQVIA Holdings, Inc.
- Laboratory Corporation of America Holdings
- Pharmaceutical Product Development, Inc.
- SGS S.A.
- Banook Group
- Biotrial Research SAS
- Certara, Inc.
- Celerion
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Koninklijke Philips N.V.
- Clario
- IQVIA Holdings, Inc.
- Laboratory Corporation of America Holdings
- Pharmaceutical Product Development, Inc.
- SGS S.A.
- Banook Group
- Biotrial Research SAS
- Certara, Inc.
- Celerion